Authors: B. Amzal, A. Movschin, A. Chiorean, J. Tanniou, M. Rollot, M. Génin
Date: 9 May 2023
CONFERENCE/VALUE IN HEALTH INFO:
N°127211, 2023-05, ISPOR 2023, Boston, MA, USA
Abstract
Objectives
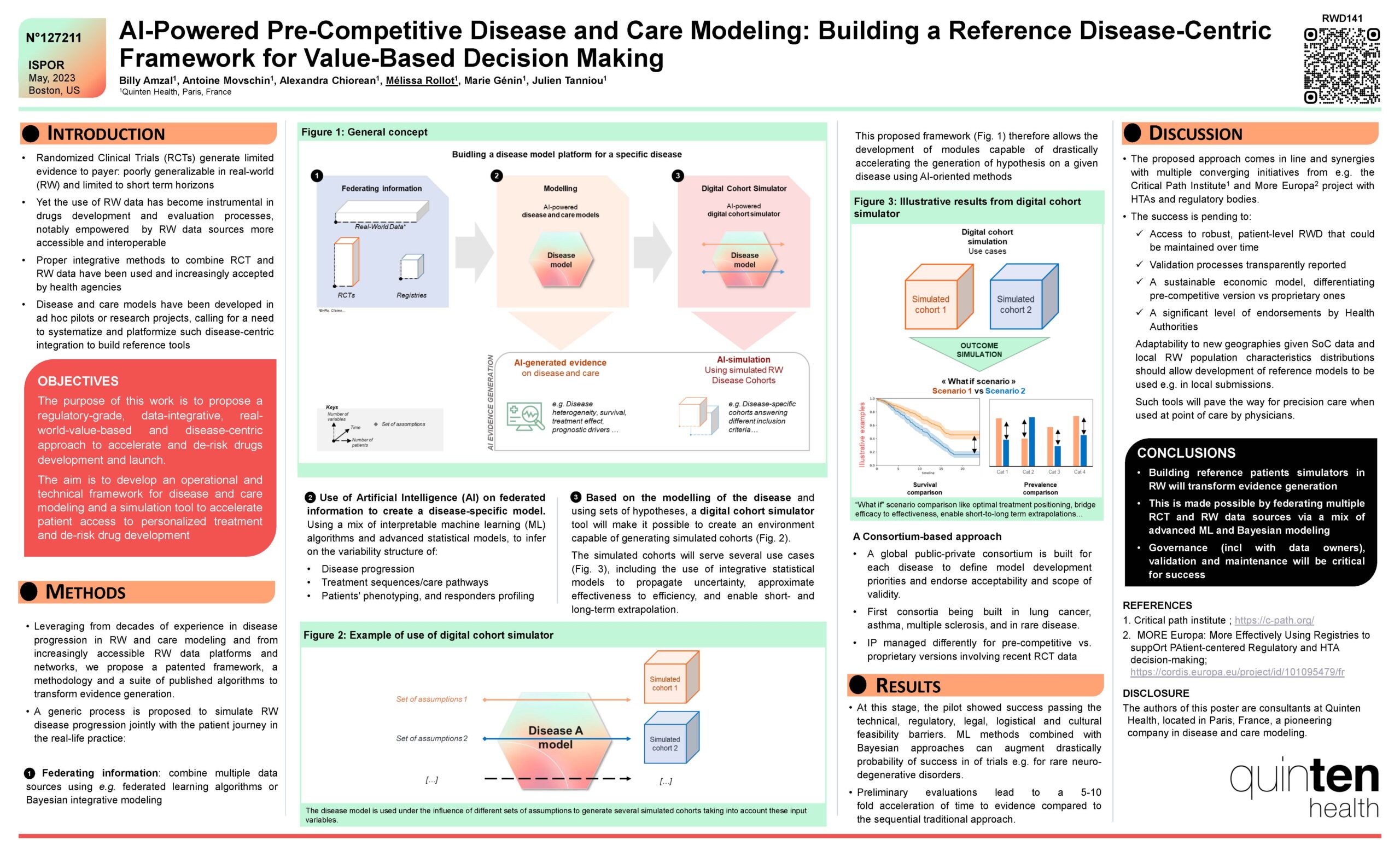
Drugs evaluation has been undergoing a transformational shift from the classical sequence of randomized control trials (RCT) and Real-World (RW) studies towards a more agile process, capitalizing better and faster from retrospective data. The purpose of this work is to propose a regulatory-grade, data-integrative, real-world-value-based and disease-centric approach to accelerate and de-risk drugs development and launch. This approach relies on disease and care modeling on the pre-competitive space and federated data sourcing.
Methods
Leveraging from decades of experience in disease progression in RW and care modeling and from increasingly accessible RW data platforms and networks, we propose a patented framework, a methodology and a suite of published algorithms to transform evidence generation. A generic process is proposed to simulate RW disease progression jointly with the patient journey in the real life practice. A mix of RW data science, Bayesian predictive modelling, interpretable machine learning (ML) is applied to characterize heterogeneity, bridge efficacy-to-effectiveness, and to enable short-to-long term extrapolations leveraging from both RCT and RW data. As an output, a reference disease cohort simulator is developed to drastically accelerate evidence generation.
A global public-private consortium is built for each disease to define model development priorities and endorse acceptability and scope of validity. First consortia were built in lung cancer, multiple sclerosis, and some rare diseases to test the concept.
Results
At this stage, the pilot showed success passing the technical, regulatory, legal, logistical and cultural feasibility barriers. ML methods combined with Bayesian approaches could be applied to multiple sources and augment drastically probability of success in trials for rare neuro-degenerative disorders.
Conclusions
Large European projects (e.g. under HORIZON) involving regulatory decision makers have been launched to test and validate such approaches. Preliminary evaluations lead to a 5-10 fold acceleration of time to evidence compared to the sequential, traditional approach.